New Metric Predicts the Likelihood of Amyloid Presence and Leads the Way in Early Alzheimer’s Detection
Novel Cognivue CARM Score Feature Now Available on Cognivue Clarity Device; Marks Milestone in Alzheimer’s Early Detection Research
May 14, 2025
ROCHESTER, N.Y. (May 14, 2025)—Neuroscience technology innovator Cognivue®, today announced a scientific breakthrough with the launch of the Cognivue Amyloid Risk Measure (CARM)*, a novel metric that predicts the likelihood of the presence of amyloid—a key biomarker of Alzheimer’s disease (AD). Now available as an optional feature on the Cognivue Clarity® device (based on FDA-cleared technology), CARM represents a significant advancement in the early detection of Alzheimer’s by helping healthcare providers identify those at risk of cognitive impairment, even before symptoms emerge.
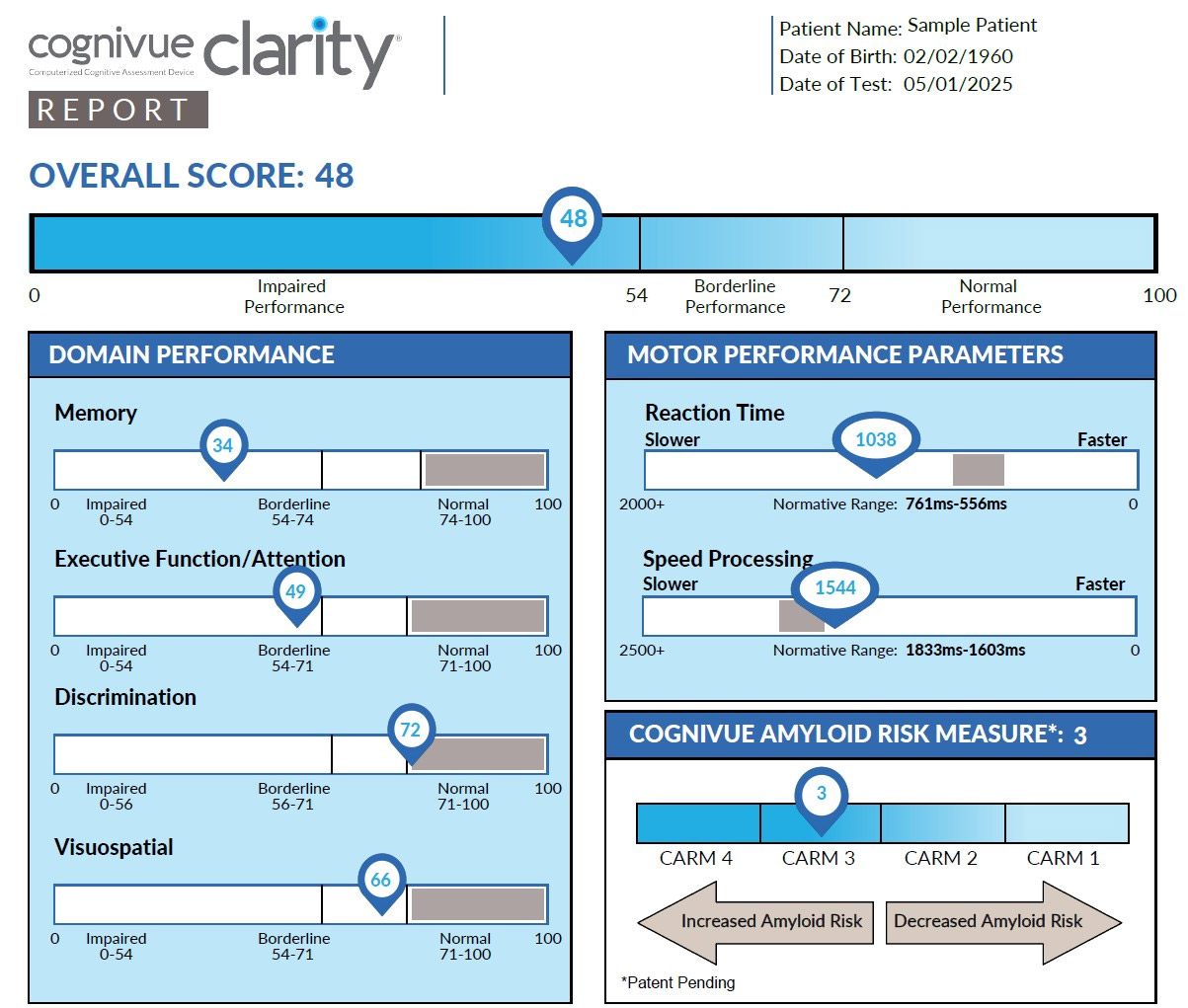
Developed using machine learning, CARM combines an individual’s age with three cognitive subtests from the Cognivue Clarity—a 10-minute digital cognitive assessment tool—to generate a four-point risk score indicating the likelihood of amyloid presence in the brain. When used alongside the Cognivue Clarity assessment, the CARM helps clinicians distinguish between normal aging, preclinical Alzheimer’s disease (pAD), AD-related cognitive impairment, and cognitive decline due to non-AD causes.1
These landmark findings and the study behind the metric, “The Cognivue Amyloid Risk Measure (CARM): A Novel Method to Predict the Presence of Amyloid with Cognivue Clarity,” were recently published in the peer-reviewed journal, Neurology and Therapy, and are available at https://link.springer.com/article/10.1007/s40120-025-00741-x.
“Alzheimer’s disease affects nearly seven million people in the U.S.,2 yet early detection remains a significant challenge—especially outside expert centers,” said James Galvin, MD, MPH, Chief Scientific Officer at Cognivue. “Cognivue Clarity addresses this critical need by providing a quick, reliable screening to evaluate cognitive function. Now, with the addition of CARM, we can estimate amyloid risk in-office—giving healthcare professionals a powerful, non-invasive tool to inform decisions and next steps.”
By predicting amyloid presence earlier as an initial screening strategy, the CARM can help clinicians identify individuals who may benefit from additional Alzheimer’s biomarker testing—such as Plasma p-tau217—supporting a more accessible and cost-effective screening pathway before pursuing PET imaging.
“At Cognivue, we’re proud to offer a solution that can help identify cognitive changes and amyloid risk earlier,” said Paul Estes, President and CEO of Cognivue. “The CARM metric builds on years of research and empowers patients and their families with actionable insights for timely intervention. Earlier detection means earlier action, which ultimately leads to better outcomes for those affected by Alzheimer’s and other forms of cognitive impairment.”
The Cognivue Amyloid Risk Measure is the latest Cognivue-led research derived from the landmark Bio-Hermes study sponsored by the Global Alzheimer’s Platform Foundation. It marks a significant step forward in advancing early detection of cognitive impairment. It is the third in a growing body of peer-reviewed research validating Cognivue Clarity’s utility in detecting and characterizing cognitive decline.
This new study builds on two previously published studies:
- “Cognivue Clarity characterizes mild cognitive impairment and Alzheimer’s disease in biomarker confirmed cohorts in the Bio-Hermes Study,” published in Scientific Reports, confirmed its ability to reliably differentiate between cognitively normal individuals, those with mild cognitive impairment (MCI), and those with mild dementia—regardless of amyloid status.3
- “Cognivue Clarity characterizes amyloid status and preclinical Alzheimer’s disease in biomarker confirmed cohorts in the Bio-Hermes Study,” published in the Journal of Alzheimer’s Disease, demonstrated Cognivue Clarity’s ability to screen for cognitive impairment, identify amyloid-positive individuals, and detect signs of preclinical Alzheimer’s disease.4
“Together, these studies offer more than scientific insight—they represent a growing body of evidence supporting Cognivue Clarity as a practical, scalable solution for cognitive assessment,” said Heather Harris, Senior Vice President of Scientific Affairs for Cognivue. “Integrated into routine care, Cognivue Clarity and CARM enable the TIER approach—identifying candidates for Treatment, Inclusion in clinical trials, Exploratory testing, and Routine cognitive screening. This streamlined assessment supports faster, more informed decisions while delivering value across the broader healthcare system.”
References:
- Galvin, J.E., Kleiman, M.J., Harris, H.M. et al. The Cognivue Amyloid Risk Measure (CARM): A Novel Method to Predict the Presence of Amyloid with Cognivue Clarity. Neurology and Therapy (2025). https://link.springer.com/article/10.1007/s40120-025-00741-x.
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dementia. 2024;2024(20):3708–821.
- Galvin, James E., Michael J. Kleiman, Paul W. Estes, Heather M. Harris, and Ernest Fung. “Cognivue Clarity Characterizes Mild Cognitive Impairment and Alzheimer’s Disease in Biomarker Confirmed Cohorts in the Bio-Hermes Study.” Scientific Reports, vol. 14, 2024, article 24519. https://www.nature.com/articles/s41598-024-75304-5.
- Galvin, James E., et al. “Cognivue Clarity Characterizes Amyloid Status and Preclinical Alzheimer’s Disease in Biomarker Confirmed Cohorts in the Bio-Hermes Study.” Journal of Alzheimer’s Disease, 2024. https://pubmed.ncbi.nlm.nih.gov/39865688/.
*Patent pending.
**Disclaimer: Cognivue Clarity® Device is indicated for use as an adjunctive tool for evaluating cognitive function. It is not a stand-alone diagnostic tool and does not identify the presence or absence of clinical diagnoses. The device results are to be assessed and interpreted by a licensed clinician.
About Cognivue, Inc.
Cognivue, Inc. is a world-class neuroscience company focusing on cognitive health with the first FDA-cleared computerized test of cognitive function. The Cognivue device and technology are based on years of research that uses adaptive psychophysics to focus on information processing by testing key cognitive domains. The technology significantly improves the ability of healthcare providers to implement a personalized assessment of cognitive function in a wide variety of care settings. The company is elevating the gold standard of cognitive health assessment and empowering the healthcare community to monitor, identify, and act early on cognitive health concerns. For more information, visit cognivue.com.
Media Contact:
Carson Daniels
McDougall Communications for Cognivue, Inc.
carson@mcdougallpr.com or +1.315.427.6394